INTRODUCTION
Over the past decade, substantial progress has been made in defining strategies for the treatment of human immunodeficiency virus (HIV) disease, the cause of acquired immunodeficiency syndrome (AIDS) , where natural products can serve as a source of structurally novel chemicals that are worth investigating as specific inhibitors of HIV as well as its essential enzymes, protease (PR) and reverse transcriptase (RT).
Ganoderma lucidum (Japanese name: Reishi) is one of the valuable
crude drugs, which has long been used in China and Japan as a traditional
Chinese medicine or a folk medicine for the treatment of various kinds
of diseases1). Several biologically active triterpenes and sterols have
been isolated from this mushroom and proved effective as cytotoxic2,3),
antiviral4) and anti-inflammatory agents5,6). Besides, polysaccharides
and glycoproteins possessing hypoglycemic7,8) and immunostimulant9-13)
activities have also been isolated from its water extract. In the course
of our continuing search for natural products as anti-HIV agents, the MEOH
extract of the fruiting bodies was found to be moderately active against
HIV-1 as well as its essential enzyme, protease (PR). Therefore this extract
was selected for further fractionation. When subjected to bioassay-guided
fractionation, the extract yielded several active compounds. This paper
describes the isolation of thirteen compounds, and their inhibitory effects
against HIV-1 and its enzyme PR.
RESULTS AND DISCUSSION
Isolation and structure determination of compounds isolated
from Ganoderma lucidum
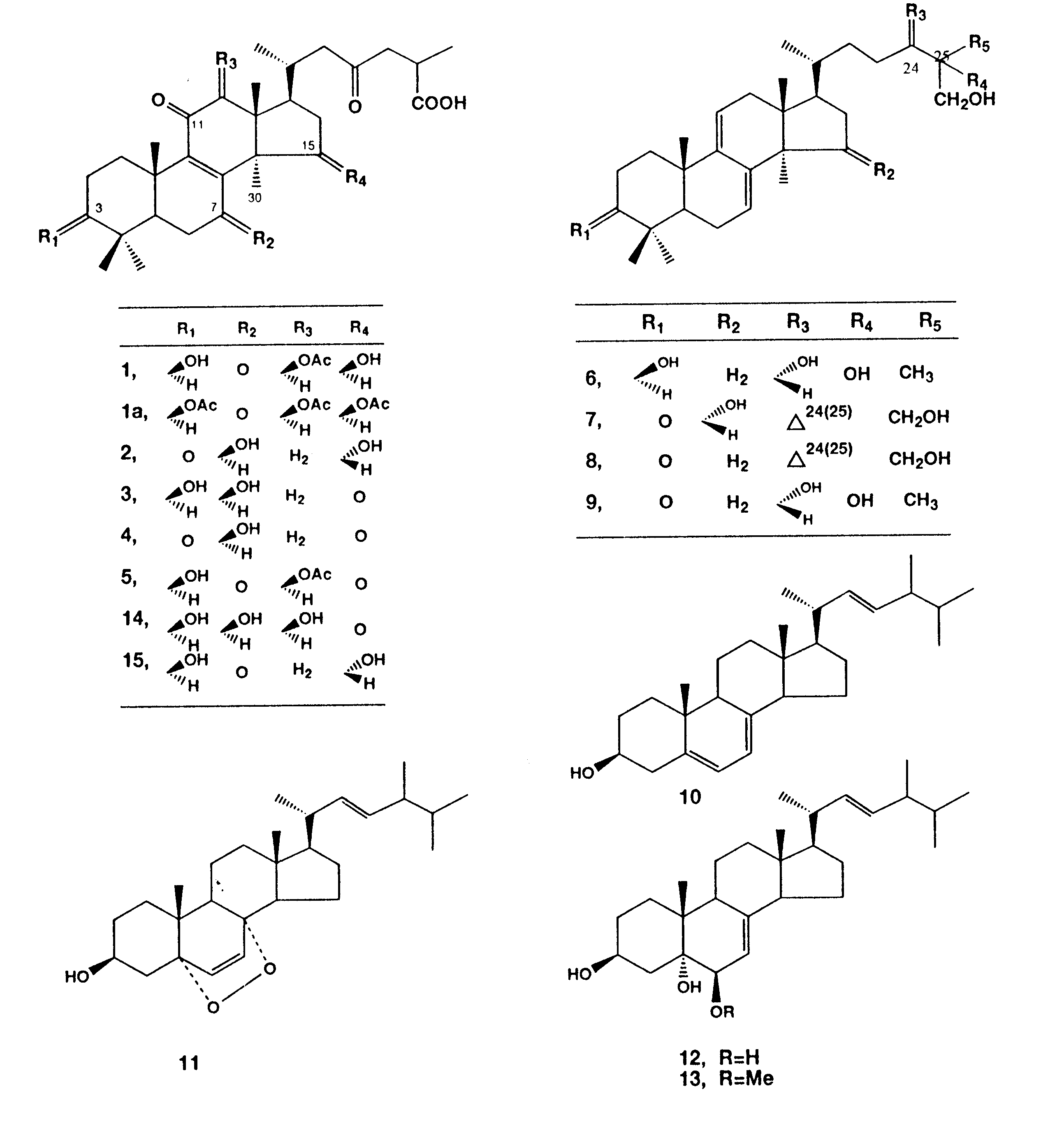
Bioactivity-guided fractionation of the MEOH extract enriched the anti- HIV and HIV-PR inhibitory effects in two fractions, B and C. Final purification of the active compounds was achieved by repeated column chromatography and HPLC to give thirteen compounds, 4, 5, 8 and 9 in fraction B, and 1-3, 6, 7 and 12 in fraction C. Three compounds (10, 11 and 13) were also obtained from fraction A. The structures of the known compounds were identified on the basis of their spectroscopic properties when compared with those reported for ganoderic acids A (2) 14-16), B (3)14-16), Cl (4)16,17) and ganoderic acid H (5)18,19), ganoderiols A (6)20) B (7)20)and F (8)21) , and ganodermanontriol (9)20), (all were previously isolated from the same mushroom). Besides, ergosterol (10), ergosterol peroxide (11, previously isolated from the sponge Ascidia nigra) 22), cerevisterol (12)23,24) and 3b -5a -dihydroxy-6b -methoxyergosta-7,22-diene (13) (both were previously isolated from the mushroom Agaricus blazei)24)
Compound 1 was obtained as an amorphous powder, [a
]D + 55.5° (CHCI3).
A molecular formula Of C32H46O9 was estimated
from a molecular ion at m/z 574 [M] +
in its mass spectrum (MS). The ultra violet (UV) absorption (254
nm) and the infrared (IR) bands (1700 and 1660 cm-1) suggested the presence
of a conjugated ketone (acid carbonyl stretching at 1750 cm-1 was also
seen). The proton nuclear magnetic resonance (1H NMR) spectrum of
1
analyzed by the aid of 1H 1H shift correlated spectroscopy (COSY) and heteronuclear
multiple quantum coherence (HMQC) experiments showed signals for
seven methyls
 |
|
|
|
Structures of compounds isolated from the MeOH extract of the fruiting bodies of Ganoderma Luciderm |
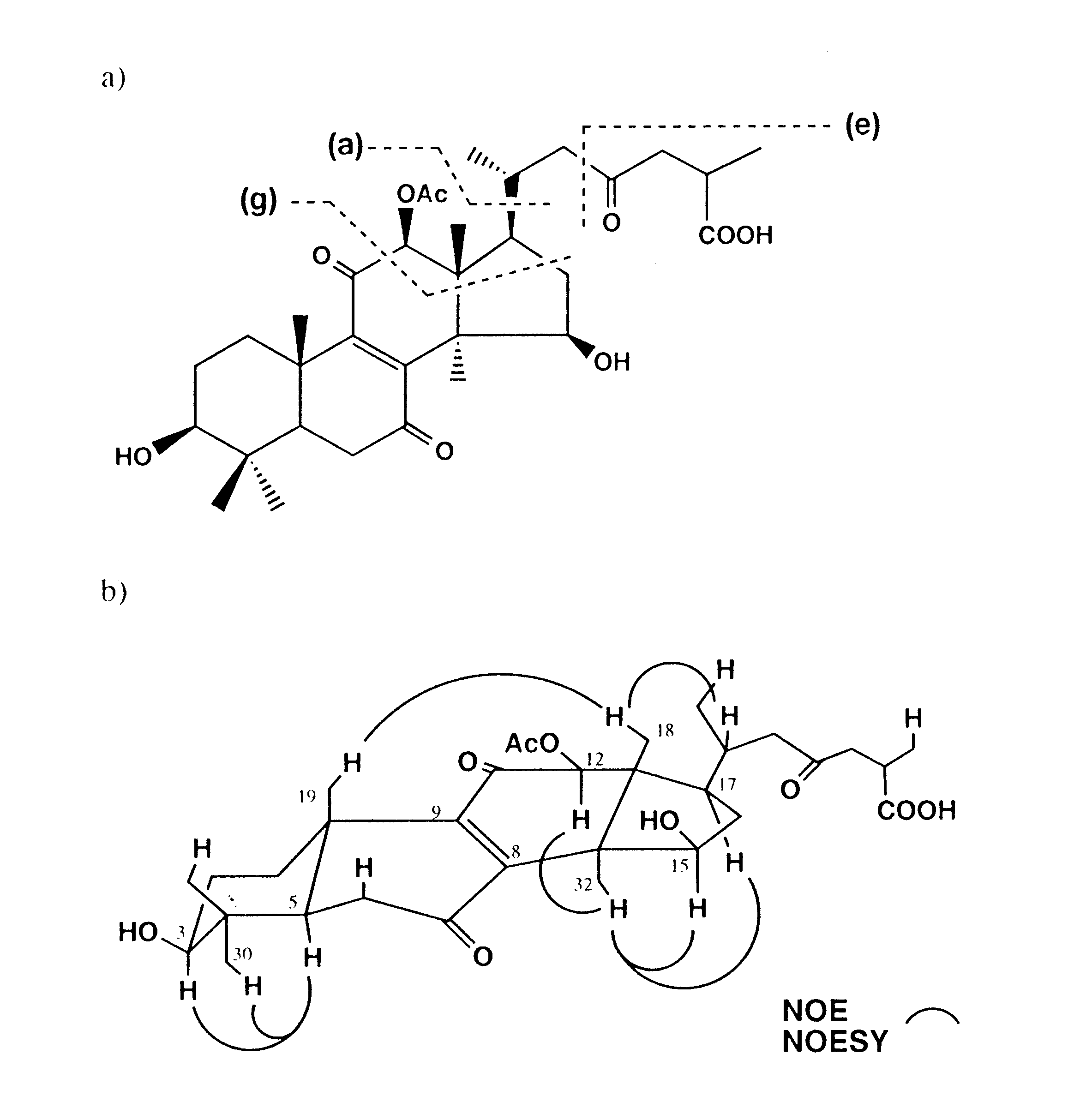
(including two as doublet at (d 0.96 and 1.22), and three methine protons at d 3.20 (dd, J = 10.5 and 5 Hz), 4.80 (dd, J = 8.5 and 4.5 Hz) and 5.62 (s). In addition, a singlet at d 2.26 for an ester methyl was also seen (Fig. 1). The carbon-13 nuclear magnetic resonance (13 C NMR) and driven equilibrium Fourier transformation (DEPT) spectra demonstrated signals characteristic for eight methyls, seven methines (including three oxymethines at d 66.2, 77.3 and 79.1), and eleven quaternary carbons (including five carbonyls at d 170.2, 179.6, 193.0, 199.0 and 206.1) (Table 1). These data suggested a highly oxygenated lanostane-type triterpene close to the respective structures of 3, 5 and ganoderic acids G (15)18), and K (16)25). However, the chemical shift difference between C-8 and C-9 (about 6.0 ppm) in 1 and 5 relative to that reported for 3 and 14 (about 16.5 ppm), suggested a substitution pattern in rings B and C similar to that of 5. The mass spectrum (Fig. 2a) displayed prominent fragment ions at m/z 513 corresponding to the loss of an acetoxyl group (as acetic acid) from the molecule, and successive losses of 18 mass units (m/z 496 and 478) indicated the presence of two hydroxyl groups. The fragment ions m/z 417 [a]+ and 115[e]+ (resulted from the cleavage between C-22 and C-23) suggested the same side chain as in related ganoderic acids.
The precise connectivity of 1 was established by interpretation of HMBC data summarized in Table 1. Long-range correlations between H-5 and C-7 (or C-9); H32 and C-8; H-19 and C-9; and H-12 and C-11 confirmed the diketone substitution at C-7 and C-11. Correlations between H-18 and C-12, and H-12 and a carbonyl carbon signal at d 170.2 (IR 1730cm-1) revealed the connectivity of the acetoxyl group at C-12. Since H-5 and H-29 were coupled to C-3, a hydroxyl group was concluded to be located at C-3. On the other hand, the 1H-1H correlations between H-15 and H16a and H-16b led to the presence of the other hydroxyl group at C-15.
The relative stereochemistry of 1 was confirmed by measuring the NOESY and nuclear Oberhauser effect (NOE) difference spectra as shown in Fig. 2b. The spatial correlations observed between H-3, H-30 and H-5 confirmed the configuration of the hydroxyl group at C-3, which was equatorially oriented (ddd, J = 10.5, 5, 5 Hz). Similarly, b -configuration of the acetoxyl group at C-12 was inferred from the correlations observed between H-12 and the proton signal at d 1.49 (H-32). Appreciable enhancement of H-15 upon irradiation of H-32, vice versa, with no evidence of spatial correlation with H-18 or long-range correlations (in 1H 1H COSY) between H-15 and H-32, confirmed the G-configuration of a hydroxyl group at C-15. Correlations between H-17 and H-32, and H-18 and a proton signal at d 2.24 (H-20) confirmed the configurations at C-17 and C-20, respectively. What remained to be established was the stereochemistry at C-25, which was
|
|
| atom 13 C 1 H HMBC |
| 1 33.1 t |
| 2 27.2 t 1.70, 1.64 |
| 3 77.3 d 3.20 ddd (10.5, 5, 5) C-2, C-5 |
| 4 40.3 s |
| 5 51.2 d 1.56 dd (1 3.5, 3.5) C-3, C-7, C-9 |
| 6 36.6 t 2.65, 2.54 C-7, C-8 |
| 7 199.0 s |
| 8 145.6 s |
| 9 151.7 s |
| 10 39.0 s |
| 11 193.0 s |
| 12 79.1 d 5.62 s C-9, C-11, C-17 |
| 13 47.9 s |
| 14 58.5 s |
| 15 66.2 d 4.80 dd (8.5, 4.5) |
| 16 48.5 t 2.46, 2.30 |
| 17 44.6 d 2.55 C-20 |
| 18 12.1 q 0.96 s C-12, C-17 |
| 19 17.9 q 1.27 s C-5, C-9 |
| 20 29.4 d 2.24 C-23 |
| 21 21.5 q 0.96 d (6) C-17, C-22 |
| 22 38.0 t 2.75, 1,92 |
| 23 206.1 s |
| 24 46.6 t 2.40, 2.80 |
| 25 35.1 d 2.91 C-23 |
| 26 179.6 s |
| 27 17.1 q 1.22 d (7) C-26 |
| 30 27.8 q 1.03 s C-3, C-5 |
| 31 15.5 q 0.85 s C-3, C-5 |
| 32 20.9 q 1.49 s C-8, C-13, C-15 |
| CH3CO 170.2 s |
| CH3CO 21.2 q 2.26 s C-12 |
suggested to be R, when compared with that of ganoderic acid H (3, given the name ganoderic acid C by Hirotani et al)25) having the same side chain which was confirmed by X-ray.
 |
|
| Fig. 2 | a) Proposed mass fragmentation
pattern of Compound 1.
b) Sterostructure for 1 as indicated by difference NOE and NOESY spectra. |
|
|
|||
| Item |
IC50 (mM) |
IC (m g/ml) |
HIV-1
|
|
MeOHext |
47.7* |
31.3# |
125# |
| Compound (1) |
|
|
|
| Ganododeric acid A (2) |
|
|
|
| Ganododeric acid B (3) |
|
|
|
| Ganoderic acid CI (4) |
|
|
|
| Ganoderic acid H (5) |
|
|
|
| Ganoderiol A (6) |
|
|
|
| Ganoderiol B (7) |
|
|
|
| Ganoderiol F (8) |
|
|
|
| Ganodermanontriol (9) |
|
|
|
| Ergosterol (10) |
|
|
|
| Ergosterol peroxide (11) |
|
|
|
| Cerevisterol (12) |
|
|
|
| 3b
-5a -dihydroxy-6-B-methoxy
ergosta-7,22-dienne (13) |
|
|
|
| IC, the minimum concentration for complete inhibition of HIV-1 induced CEP in MT-4 cells by microscopic observation. CC, the minimum concentration for appearance of MT-4 cell toxicity by microscopic observation. NE, not effective. ( ) , concentration at which weak anti-HIV-1 activity was observed.* %Inhibition at 100m g/ml. #As m g/ml | |||
Inhibitory effects of isolated compounds on HIV and its enzymes
Investigation of anti-HIV and PR-inhibitory activities of the isolated compounds (1-13) yielded some compounds with moderate activities (Table 2). In the primary screening test for anti-HIV activity, compounds 8 and 9 were found to inhibit HIV1 induced cytopathic effect (CPE) in MT-4 cells with a 100% inhibitory concentration (IC) value of 7.8 m g/ml for both compounds, and the IC value for both was a half of the respective cytotoxic concentration (CC) value.
As for HIV-1 PR inhibitory effects, the PR activity was determined by analysing the hydrolysates of a synthetic substrate in the presence or absence of the isolated compounds using high performance liquid chromatography (HPLC) method. Of the tested compounds, 3 and 7 were found to be the most active against HIV-1 PR enzyme with an IC50 of 0.17 mM for both compounds. Other compounds such as ganoderiol B, ganoderic acid Cl, 3b -5a -dihydroxy-6,b -methoxyergosta-7,22-diene, compound 1, ganoderic acid H and ganoderiol A inhibited the enzyme activity in a similar extent.
In the present experiment, we found that D7(8),D 9(11)-lanostadiene-type triterpenes had relatively strong anti-HIV activity. On the other hand, D8(9) -lanostene-type triterpenes and ergostane-type compounds 10-12 had no inhibition of HIV-induced cytopathic effects. As to HIV-protease, we could not obtain any conclusive findings on the structure-activity relationship. Lanostane-type triterpenes showed IC50 of 0.17-0.32 mM, while ergosterol derivatives had no inhibitory activity. However, it was reported that synthetic cosalane and its derivatives had an anti-HIV effect as well as inhibitory effects on RT and PR27) . Several triterpenes have been described as antiviral compounds. Glycyrrhizin displays some limited activity against a whole range of viruses including HIV-128). Salaspermic acid29) and suberol (a lanostane-type)30) inhibit HIV-1 in H9 cells in the upper micromolar range. Bile acid derivatives were found slightly active (at 10-4 M) against HIV-1 in MT-4 cells31). Betulinic acid derivatives (lupane-type) have been described as potent inhibitors of the cytopathogenicity of HIV-1 in CEM 4 and MT-4 cells without affecting HIV-1 RT or PR activity32).
When compared with other triterpenes reported, compounds 8 and 9 can be used as leads to develop other related compounds with potential anti-HIV activity. This subject will be of particular interest to be investigated in the future.
REFERENCES
2. Toth, J. 0., Luu, B. and Ourisson, G. (1983) Tetrahedron Letters 24, 1081-1084.
3. Kohda, H., Tokumoto, W., Sakamoto, K., Fujii, M., Hirai, Y., Yamasaki, K., Komoda, Y., Nakamura, H. Ishihara, S. and Uchida, M (1985) Chem. Pharm. Bull. 33, 1367-1373.
4. Lindequist, U., Lesnau, A., Teuscher, E. and Pilgrim, H. (1989) Pharmazie 44, 579-580.
5. Tasaka, K., Akagi, M., Miyoshi, K., Mio, M. and Makino, T. (1988) Agents Actions 23, 153-156.
6. Tasaka, K., Mio, M., lzushi, K., Akagi, M. and Makino, T. (1988) Agents Actions 23, 157-160.
7. Hikino, H. and Mizuno, T. (1989) Planta Medica 55, 358.
8. Hikino, H., Ishiyama, M., Suzuki, Y. and Konno, C. (1989) Planta Medica 55, 423-428.
9. Lei, L., S. and Lin, Z.,B. (1993) Yao-Hsueh-Hseuh-Pao 28, 577-582.
10. Lei, L., Lin, Z., Chen, Q., Li, R. and lie, Y. (1993) Zhongguo Yaolixue Yu Dulixue Zashi 7, 183.
11. Kino, K., Yamashita, A., Yamaoka, K., Watanabe, J., Tanaka, S., Ko, K., Shimizu, K. and Tsuno, H. (1989) J. Biol. Chem. 264, 472-478.
12. Kino, K., Sone, T., Watanabe, J., Yamashita, A., Tsuboi, H., Miyama, H and Tsuno, H. (1985) Int. J. Immunopharmacol. 13, 109-1115.
13. He, Y., Li, R., Chen, Q., Lin, Z., Xia, D. and Ma, L. (1992) d. Chin. Pharm. Sci. 1, 79-81.
14. Kubota, T., Asaka, Y., Miura, 1. and Mori, H. (1982) Helv. Chim. Acta 65, 611-619.
15. Kikuchi, T., Matsuda, S., Murai, Y. and Ogita, Z. (1985) Chem. Pharm. Bull. 33, 2628-
16. Kikuchi, T., Kanmi, S., Kadota, S., Murai, Y., Tsubuno, K. and Ogita, Z. (1986) Chem. Pharm. Bull. 34, 3695-3712.
17. Nishitoba, T., Sato, H., Kasai, T., Kawagishi, H. and Sakamura, S. (1985) Agric. Biol. Chem. 49, 1793-1798.
18. Kikuchi, T., Kanmi, S., Kadota, S., Murai, Y., Tsubuno, K. and Ogita, Z. (1986) Chem. Pharm. Bull. 34, 4018-4029.
19. Kikuchi, T., Matsuda, S., Murai, Y. and Ogita, Z. (1985) Chem. Pharm. Bull. 33, 26242627.
20. Sato, H., Nishitoba, T., Shirasu, S., Oda, K. and Sakamura, S. (1986) Agric. Biol. Chem. 50, 2887-2890.
21. Nishitoba, T., Oda, K., Sato, H., and Sakamura, S. (1988) Agric. Biol. Chem. 52, 367-372.
22. Gunatilaka, A. A. L., Gopichand, Y., Schmitz, F. J. and Djerassi, C. (1981) J. Org. Chem. 46, 3860-3866.
23. Iorizzi, M., Minale, L. and Riecio, R. (1988) J. Nat. Prod. 51, 1098-1103.
24. Kawagishi, H., Katsumi, R., Sazawa, T., Mizuno, T., Hagiwara, T. and Nakamura, T. (1988) Phytochemistry 27, 2777-2779.
25. Morigawa, A., Kitabataki, K., Fujimoto, Y. and Ikekawa, N. (1986) Chem. Pharm. Bull. 34, 3025-3028.
26. Hirotani, M., Furuya, T. and Shiro, M. (1985) Phytochemistry 24, 2055-2061.
27. Cushman, M., Golebiewski, W. M., MeMahon, J. B., Buckheit, R. W. J., Clanton, D. J., Weislow, 0., Haugwitz, R. D., Bader, J., Graham, L. and Rice, W. G. (1994) J. Med. Chem. 37, 4030-3050.
28. Pompei, R., Flore, 0., Marccialis, M. A., Pani, A. and Loddo, B. (1979) Nature 218, 689-690.
29. Chen, K., Shi, Q., Kashiwada, Y., Zhang, D.-C., Hu, C.-Q., Jin, J.-Q., Nozaki, H., Kilkuskie, R. E., Tramontano, E., Cheng, Y. C., MaPhail, D. R. and Lee, K.-H. (1992). Nat. Prod. 55, 340-346.
30. Li, H.-Y., Sun, N.-J., Kashiwada, Y., Sun, L., Snider, J. V., Cosentino, L. M. and Lee, K.-H. (1993) J. Nat. Prod. 56, 1130-1133
31. Baba, M., Schols, D., Nakashirna, ll., Pauwels, R. Parmentier, G. Meijer, D. K. F. and DeClercq, E. (1989) J. Acquired Immune Defic. Syndr. 2, 264-271.
32. Evers, M., Poujade, C., Soler, F., Ribeill, Y., James,
C., Lelievre, Y., Gueguen, J.-C., Reisdorf, D., Morize, I., Pauwels, R.,
DeClercq, E., Henin, Y., Bousseau, A., Mayaux, J.-F., LePecq, J.-B. and
Dereu, N. (1996) J. Med. Chem. 39, 1059-1068.
 |
Masao
Hattori
Ph. D. Professor of Toyama Medical and Pharmaceutical University Born on 21th, April, 1944
Education: March, 1967 Graduated fromOsaka University, Osaka, Japan March, 1969 Finished the Master course March, 1972 Finished the Doctor courseReceived Ph. D from Osaka University |
| March, 1972-March,
1975 Visiting fellow of National Institute
of Health, Bethesda, Maryland, U. S. A.
July,1975-Oct. 1977 Visiting fellow of Konstanz University,Konstanz Germany Sept. 1977-April. 1979 Special research fellow of National Institute of Genetics,Mishima, Japan May, 1979-Jan. 1991 Associate Professor of Toyama Medical and Pharnaceutical University, Toyama Feb. 1991- Professor of Toyama Medical
and Pharmaceutical University
|
|